Sinopharm Vaccine Who Approval
The National Primary Health Care Development Agency NPHCDA said NAFDAC had approved Sinopharm BBIBP-CorV COVID-19 vaccine to step up efforts to battle a third wave of infections in the country. 2 days agoThe Sinopharm and Sinovac Covid-19 vaccines have been added to Saudi Arabias list of approved vaccines bringing the total number to six.
Coronavirus Live China S Sinopharm Jab Gets Who Approval Turkey Maldives And Nepal Added To England Red List World News The Guardian
National regulatory authorities have granted emergency use authorizations for twenty-two COVID-19 vaccinesSix of those have been approved for emergency or full use by at least one WHO-recognized stringent regulatory authority OxfordAstraZeneca Pfizer-BioNTech Sinopharm-BBIBP Moderna Sinovac and.
Sinopharm vaccine who approval. Sinopharm joins Sinovac AstraZeneca Johnson Johnson and Moderna to be officially approved for use in Thailand. Here is what you need to know. And yes Sinopharm vaccine has also received WHO certification Shuaib told a press conference in Abuja on Tuesday.
The National Primary Health Care Development Agency NPHCDA said the National Agency for Food and Drug Administration and Control NAFDAC had approved Sinopharm BBIBP-CorV COVID-19 vaccine to step up efforts to battle a third wave of infections in the country. Today Sinopharm was approved shortly after it was revealed that Princess Chulabhorn had negotiated a private batch of 1 million Sinopharm vaccines for the Chulabhorn Royal Acadamy that she sponsors set to arrive next month. Sinopharm Vaccine Approved By HSA Under Special Access Route.
Speaking on Tuesday Shuaib said the National Agency for Food and Drug Administration and Control NAFDAC approved the vaccine three days ago. NAFDAC has approved Sinopharm vaccine. Sinopharm Covid-19 Vaccine The Executive Director NPHCDA Dr.
Faisal Shuaib said this on Tuesday August. The National Primary Health Care Development Agency NPHCDA said NAFDAC had approved Sinopharm BBIBP-CorV COVID-19 vaccine to step up efforts to. NAFDAC has approved Sinopharm vaccine the approval was done three days ago.
The Sinopharm vaccine is produced by Beijing Bio-Institute of Biological Products Co Ltd subsidiary of China National Biotec Group CNBG. Beijings Covid vaccine is. The Executive Director NPHCDA Dr.
Nigerian govt approves Sinopharm vaccine. The World Health Organization said it has approved for emergency use a coronavirus vaccine developed by the Chinese state-owned pharmaceutical group Sinopharm. Executive Director of National Primary Health Care Development Agency NPHCDA Faisal Shuaib made the disclosure.
The World Health Organization WHO has granted emergency approval for a Covid vaccine made by Chinese state-owned company Sinopharm. Faisal Shuaib said this on Tuesday in Abuja at the National Vaccines briefing. So it is a potential vaccine that we could use he added.
2 days agoOn 7 May 2021 the World Health Organization approved the vaccine for use in COVAX. NEWS DIGEST The National Primary Health Care Development Agency NPHCDA said NAFDAC had approved Sinopharm BBIBP-CorV COVID-19 vaccine to step up efforts to battle a third wave of infections in the country. The Executive Director NPHCDA Dr.
Faisal Shuaib said this on Tuesday in Abuja at the National Vaccines. A vaccination certificate of BBIBP-CorV Beijing Institute of Biological Products Sinopharm. The Sinopharm COVID-19 vaccine is an inactivated COVID-19 vaccine and had been approved by the World Health Organisation WHO for emergency use.
The National Agency for Food and Drug Administration and Control NAFDAC has approved the use of the Sinopharm BBIBP-CorV COVID-19 vaccine in the country. According to the Saudi Gazette the Saudi Ministry of Health has approved six vaccines Oxford-AstraZeneca Pfizer-BioNTech Johnson Johnson Moderna Sinopharm and Sinovac. The World Health Organisation approved a Covid-19 vaccine from Chinas state-owned drugmaker Sinopharm for emergency use on Friday a boost to Beijings push for a big role in.
The other inactivated virus vaccine developed by Sinopharm is WIBP-CorV. Mexico grants emergency use approval for Chinas Sinopharm vaccine Mexican navy helicopter makes crash landing no deaths reported SCOTUS Stops Joe Biden From Removing Trump Remain In Mexico. Shuaib who made the announcement during a press briefing on Tuesday August 24 said the National.
Sinopharm has signed purchase agreements for 170 million doses from COVAX. SINGAPORE The Health Sciences Authority HSA has approved multiple applications for private hospitals or clinics to bring in the Chinese-made Sinopharm vaccine. The WHO Strategic Advisory Group of Experts SAGE on Immunization has issued Interim recommendations for the use of the inactivated COVID-19 vaccine BIBP developed by SinopharmChina National Pharmaceutical Group.
Even though the Pfizer-BioNTech and Moderna vaccines are available for free under our national vaccination programme some may look for alternative options. The Sinovac vaccine in particular is extremely popular with nearly 100000 doses administered to date. It is the first vaccine developed by a.
The Federal Government has approved Sinopharm COVID-19 vaccine.

Who Panel Oks Emergency Use Of China S Sinopharm Vaccine Paving Way For Doses To Reach Needy Countries Ktla
Sinopharm A Lifeline To Nations Without Easy Access To West S Vaccines Cgtn
Pakistan Approves Chinese Sinopharm Covid 19 Vaccine For Emergency Use Reuters
Who Approval Of Sinopharm Vaccine Set To Benefit World Coronavirus Fight World Chinadaily Com Cn

Hungary Reaches Deal To Buy China S Sinopharm Vaccine Euractiv Com

Who Approves China S Sinopharm Covid 19 Vaccine For Emergency Use Has 79 Efficacy Coronavirus Outbreak News

China Picks Uae To Make Millions Of Vaccines Boosting Gulf Ties Bloomberg
China Helps Serbia Surge Ahead In European Vaccine Race

Sinopharm S Covid 19 Vaccine Is 86 Effective According To Uae Regulators Drug Discovery And Development
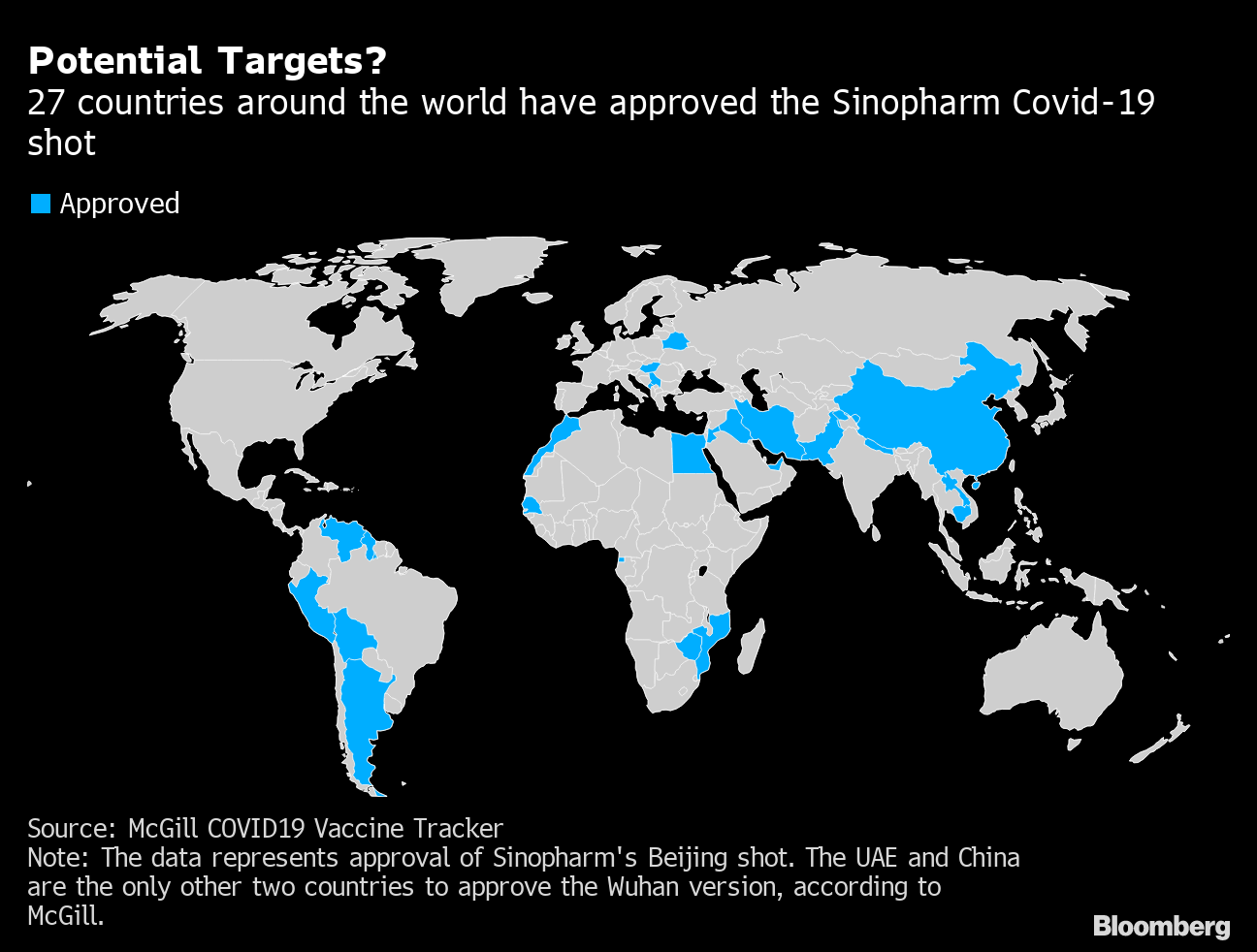
China Picks Uae To Make Millions Of Vaccines Boosting Gulf Ties Bloomberg

China State Backed Covid Vaccine Has 86 Efficacy Uae Says Bloomberg

Chinese Covid 19 Vaccine Sinopharm Gets Who Approval For Emergency Use Businesstoday
Argentina Approves Emergency Use Of Sinopharm Vaccine

Sinopharm Vero Cell Inactivated Covid 19 Vaccine

China State Backed Covid Vaccine Has 86 Efficacy Uae Says Bloomberg

China To Gift Pakistan 500k Covid 19 Vaccine Doses Voice Of America English
Sinopharm In Center Of Professional And Political Debate After Reports Question Effectiveness

Who And Ema Approved Vaccine List Which Covid 19 Vaccines Are On Who And Ema List Updated 25 August 2021 Wego Travel Blog

Sinopharm S Wuhan Institute Rolls Out Its First Batch Of Covid 19 Vaccines For Mass Use Annual Output Reaches 100 Million Doses Global Times
Post a Comment for "Sinopharm Vaccine Who Approval"